Research
Research Program and Highlights
Overall, my research is broadly aimed at understanding the origin of the distribution of elements in the solar system, with emphasis on the Earth and terrestrial planets. This work is largely focused on experimental investigation of interphase partitioning, phase stability and the kinetics of element exchange. Current and near-future research is to develop experimental and analytical techniques to better assess the role of magmatic processes in controlling the behaviour of the highly siderophile elements: Re, Au and the Platinum Group Elements (PGEs; Os, Ir, Ru, Rh, Pt, Pd). Current work in that area is to assess the capacity for carbonate melt to dissolve these elements, along with sulfur, and the effects of carbonatite metasomatism. Experiments are also in progress to measure the solubility of sulfur in high Fe melts at reduced conditions, and thereby determine if the lunar mantle is sulfide saturated. I also have a longstanding interest in the nature of geochemical recycling at convergent margins, and most recently have become involved in research on the record of early Earth processes. Specific recent and ongoing research projects, with a description of research accomplishments, involving myself, students and other collaborators are detailed below (in roughly chronological order).
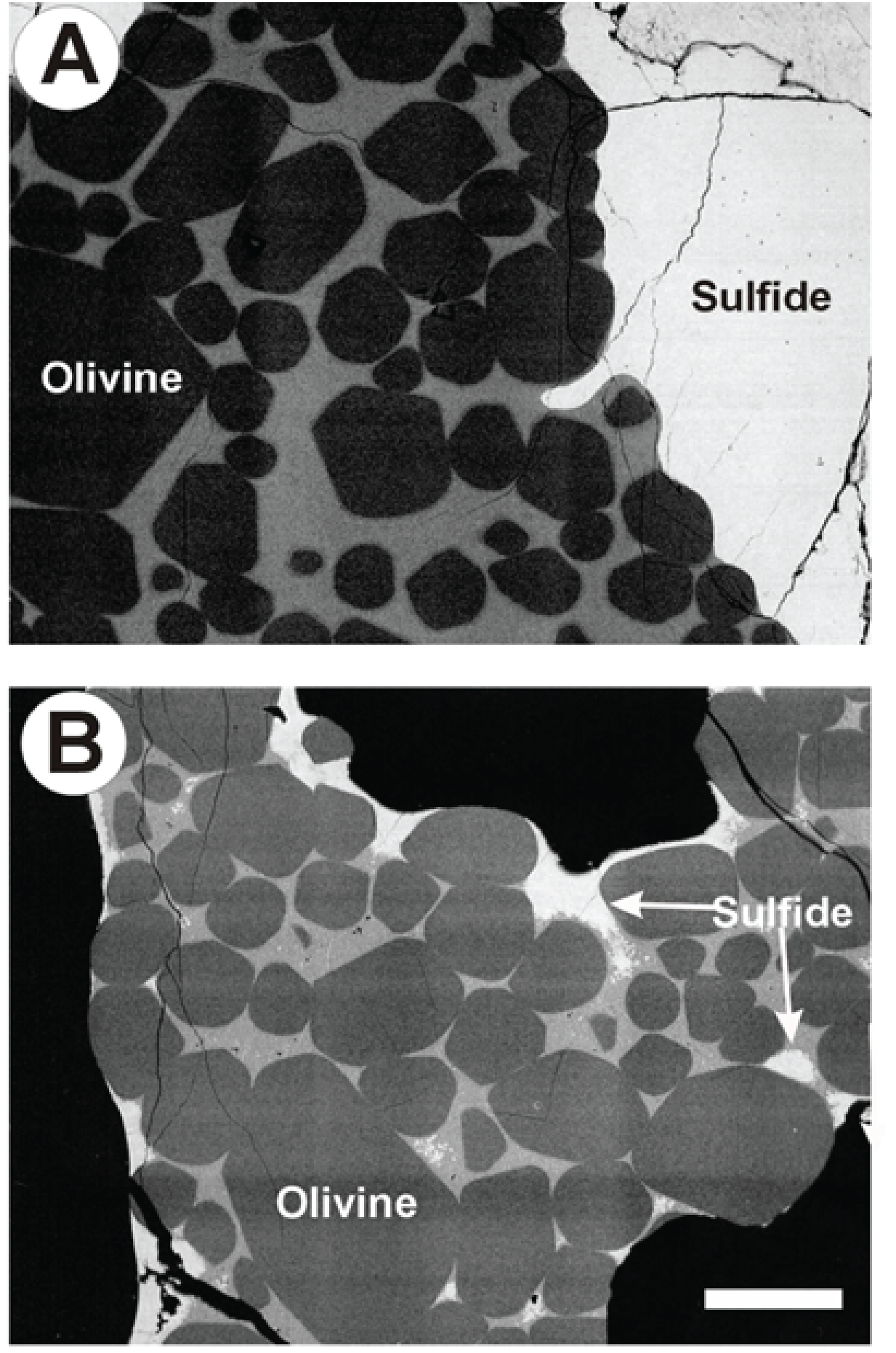
A. Olivine-sulfide melt Fe-Ni exchange and the origin of magmatic Ni deposits
There has been a longstanding debate regarding the exact mechanism for the origin of some sulfide-hosted copper-nickel-PGE deposits associated with mafic and ultramafic igneous rocks. Evidence exists to suggest either concentration by an immiscible sulfide melt at the magmatic stage, or by syn- to post-solidification hydrothermal fluids. One of the primary pieces of evidence that has been used to dispel the magmatic model is that, based on comparison with existing experimental Fe and Ni partitioning data, there is an apparent lack of equilibrium between olivine (the primary liquidus phase in these rocks) and coexisting sulfide. The experimental database used to support this contention is very limited, however, and until recently, no comprehensive study has documented the effects of melt composition, oxygen fugacity or temperature on olivine-melt partitioning. Natalie Caciagli (at the time, a B.Sc. student) and I initiated a series of experiments to measure sulfide-olivine partitioning of Ni, which was followed up by a more extensive study done by myself. Our results showed that the Fe-Ni exchange between olivine and sulfide melt is a strong function of the Fe/Ni ratio of the melt and oxygen fugacity. Taking these effects into account, the compositions of coexisting olivine and sulfide from a variety of mafic and ultramafic igneous rocks have now been shown to be consistent with equilibrium at oxygen fugacities reasonable for terrestrial igneous rocks. These results are also the first to provide a means for directly estimating the ambient oxygen fugacity during ore formation, which may shed new light on the ore formation process. Recent work in this theme in collaboration with Steve Barnes and colleagues at CSIRO (Australia) has provided a regression of the Ni partitioning results, which has been used to explain the origin of extreme Ni-enrichment in some sulfide ores.
Relevant publications
1) Brenan, J.M. and Caciagli, N Fe-Ni exchange Between Olivine and Sulphide Liquid: Implications for oxygen barometry in sulphide-saturated magmas Geochimica et Cosmochimica Acta, 64:307-320, 2000
2) Brenan, J.M. Effects of fO2, fS2, Temperature and Melt Composition on Fe-Ni Exchange Between Olivine and Sulfide Liquid: Implications for Natural Olivine-Sulfide Assemblages, Geochimica et Cosmochimica Acta, 67: 2663-2681, 2003.
3) Barnes, S.J., Godel, B., Gürer, D., Brenan, J.M., Robertson, J. and Paterson, D., Sulfide-olivine Fe-Ni exchange and the origin of anomalously Ni-rich magmatic sulfides. Economic Geology, 108, pp 1971-1982, 2013.
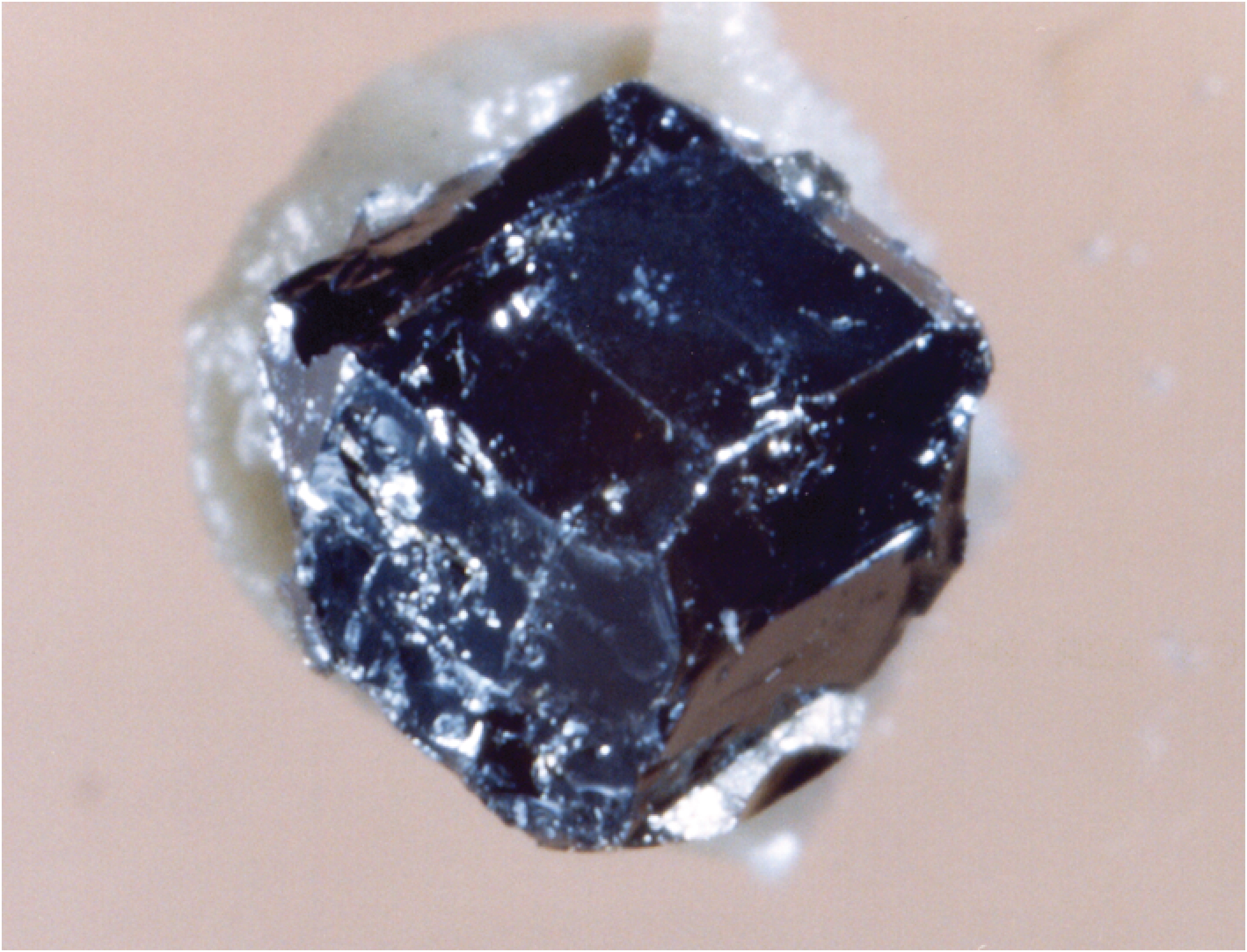
B. Stability and origin of platinum group minerals (PGMs)
The magmatic origin of PGE-rich accessory minerals such as laurite (RuS2) and Ru-Os-Ir alloys has been subject to some uncertainty, but bears directly on the mechanisms of PGE concentration and fractionation in mafic igneous systems. Dave Andrews (M.Sc., 2002) and I conducted an extensive series of experiments to document the high temperature stability of some these minerals, as well as their solubility in molten sulfide. Our results showed that laurite is refractory, and that the composition of coexisting natural laurite and Ru-Os-Ir alloy is consistent with the sulfur fugacity recorded by mafic magmas at near-liquidus conditions, thus supporting a high temperature origin for these phases. We also demonstrated that Ru can be significantly soluble in molten sulfide, which combined with a diminishingly small solubility in silicate, implies sulfide-silicate partitioning of 107 or greater, which is at least 1000x higher than previous estimates. This result emphasizes the enormous extraction capacity of sulfide liquids for PGE, and the importance of the sulfide/silicate mass ratio in producing significant PGE concentrations in natural systems. These large partition coefficients have since been confirmed by direct measurements on coexisting sulfide and silicate melt published by Jim Mungall and myself (Mungall and Brenan, 2014; see project e). Another prediction of the PGM work is that komatiite, which is generated by high degrees of melting, with subsequently high PGE content, should be saturated in Ru-bearing (+ Ir and Os) alloy. Work reporting new data on the Ir, Ru and Rh content of komatiites suggests behaviour consistent with an alloy saturation surface, similar to chromite, confirming our prediction.
Future experiments in this area will be to investigate the remarkable longevity of platinum- and osmium-iridium-rich accessory minerals which have been recently discovered to be ubiquitous in upper mantle samples. Not only are these minerals likely to control the abundance of platinum-group elements in mafic magmas, but they may also be amongst the most primitive solids in the Earth. Some of these grains have ages which exceed 2.5 Ga, and it is possible older grains may yet to be found. This is an astounding degree of preservation given the high ambient temperatures these samples of been exposed to. Their ancient ages are intriguing, and questions arise as to how they formed. Few constraints exist to evaluate the extent to which these grains may be preserved in a convecting mantle, including the degree to which they may be dissolved in mantle sulfides, and the temperatures and time-scales required to reset their internal isotopic clocks.
Relevant publications
1) Andrews, D.R.A. and Brenan, J.M. Phase Equilibrium Constraints on the Magmatic Origin of Laurite + Ru-Os-Ir Alloy, Canadian Mineralogist, 40: 1705-1716, 2002.
2) Andrews, D.A. and Brenan, J.M. The Solubility of Ruthenium in Sulphide Liquid: Implications for Platinum-Group Mineral (PGM) Stability and Sulphide Melt/Silicate Melt Partitioning, Chemical Geology,192:163-181, 2002.
3) Brenan, J.M. and Andrews, D. High Temperature Stability of Laurite and Ru-Os-Ir Alloy and Their Role in PGE Fractionation in Mafic Magmas, Canadian Mineralogist, 39:573-592, 2001.
C. Halogens in sulfide systems
Unusual abundances of halogen-rich minerals are found in close spatial association with base and precious metal sulfide mineralization cogenetic with igneous rocks in several localities worldwide. A contentious issue is whether this paragenesis represents deposition at the magmatic stage, or is the result of later deuteric or hydrothermal processes. Jim Mungall and I collaborated on experiments to measure the partitioning of halogens between molten silicate and sulfide, as well as between monosulfide solid solution and molten sulfide. Our results are the first to show that sulfide magmas are capable of dissolving and transporting significant concentrations of halogens in the absence of aqueous fluids, indicating that observed enrichments in halogens in some mineralized environments are consistent with ore deposition by purely magmatic processes.
Relevant publications
1) Mungall, J. E. and Brenan, J.M. Experimental Evidence for the Chalcophile Behaviour of the Halogens, Canadian Mineralogist, 41: 207-220, 2003.
This contribution won the Hawley Medal for the best paper published in Canadian Mineralogist for 2003.
D. Olivine-melt partitioning of platinum group elements (PGEs)
A significant goal of the research program I initiated at University of Toronto was to provide a basic understanding of the phases which control the concentration and fractionation of the PGEs, Re and Au in magmatic systems. Olivine is one mineral that has been repeatedly implicated as a reservoir for the so-called IPGEs (Os, Ir and Ru), and was thought to be an important phase in producing the high Re/Os ratio that typifies crust-forming melts. Hence, our initial experiments focused on olivine, with considerable work done on both experimental methods, and analytical protocols, in collaboration with Prof. Bill McDonough at University of Maryland. Techniques developed during those initial studies have since been applied to measure chromite-melt partitioning, as well as metal solubility at high P and T. Our results for olivine showed that the IPGEs are compatible in olivine, whereas Pt, Pd, Re and Au are excluded, indicating that this mineral contributes to PGE and Re-Os fractionation during mantle melting, or crystallization of mafic melts, in the absence of a sulfide phase. Results also show that the solubility of Os, Ru and Ir in molten silicate is very low, and comparable to the concentrations of these elements in primitive, sulfur-poor magmas, suggesting they could be metal-saturated. Those results were amongst the first to document the intrinsic mineral/melt partition coefficients for Au, Re and the PGEs, and clearly underscores the importance of silicate minerals, in addition to sulphide and alloy phases, in controlling the behaviour of these elements under some conditions.
Relevant publications
1) Brenan, J.M., McDonough, W.F. and Dalpe, C., Experimental Constraints on the Partitioning of Rhenium and Some Platinum-Group Elements between Olivine and Silicate Melt, Earth and Planetary Science Letters, 212:135-150, 2003
2) Brenan, J.M., McDonough, W.F. and Ash, R. An experimental study of the solubility and partitioning of iridium, osmium and gold between olivine and silicate melt. Earth and Planetary Science Letters, 237:855-872, 2005.
E. Re-Os and PGE fractionation during crust-mantle differentiation
Primitive mafic magmas from different tectonic settings are characterized by superchondritic Re/Os values which are long-lived in crustal rocks and have given rise to the very large difference in Os isotopic composition between the crust and mantle. Undepleted mantle peridotites have near-chondritic relative abundances of these elements implying that Os is significantly more compatible than Re during partial melting. Previous laboratory experiments and data from natural samples show that sulfide melt -silicate melt partitioning of Os (and other PGEs) can be 10,000 or larger, which is adequate to produce the Os concentrations in primitive mantle-derived magmas. However, an apparent paradox arises when attempting to reconcile the relative partitioning of Re and Os in the presence of residual sulfide. The main problem is the very large disparity in previous estimates of the sulfide melt-silicate melt partitioning for Re, with values ranging from ~20,000 to ~40. The value of 20,000 is too large to account for the mildly incompatible behaviour of Re in mantle-derived magmas, except in the presence of a diminishingly small amount of residual sulfide. To resolve this dilemma, sulfide-silicate partitioning of Re and Os was explored in experiments done over a range of fO2-fS2 conditions using internal buffering techniques and a novel high temperature centrifuge furnace to promote in situ sulfide-silicate separation. An important discovery was that the sulfide-silicate partition coefficient for Re decreases dramatically with increased fO2, at constant fS2, whereas values for Os remain uniformly large. Results show that at the fO2-fS2 conditions of terrestrial magmagenesis, Re is significantly more incompatible than Os in sulfide-bearing mantle residues, thus accounting for the Re-Os fractionation during crust-mantle differentiation. In collaboration with Jim Mungall, we have now applied similar techniques to measure the sulfide-silicate partitioning of all the PGEs and Au. Results of that work revealed partition coefficients that exceed 106, which is significantly larger than most previous measurements which involved bulk analytical methods, although consistent with estimates based on metal solubility (see section b). Two important implications of that work are that 1) the PGEs will become highly concentrated in the small fraction of sulfide liquid remaining during melting, possibly leading to the formation of accessory minerals that concentrate these elements (Os-Ir and Pt alloys, RuS2 – laurite) accounting for their presence in refractory mantle rocks and 2) the very large enrichments of the PGEs in rocks such as the Merensky Reef are explicable in the context of small amounts of sulfide produced from magmas with relatively unremarkable PGE-levels.

Relevant publications
1) Brenan, J.M. Re-Os fractionation by sulfide-silicate partitioning: A new spin. Chemical Geology, Special Issue on Highly Siderophile Elements, vol 248, pp 140-165, 2008.
2) Mungall, J. E, and Brenan, J.M. Partitioning of platinum-group elements and Au between sulfide liquid and basalt and the origins of mantle-crust fractionation of the chalcophile elements. Geochimica et Cosmochimica Acta, 125 pp 265-289, 2014
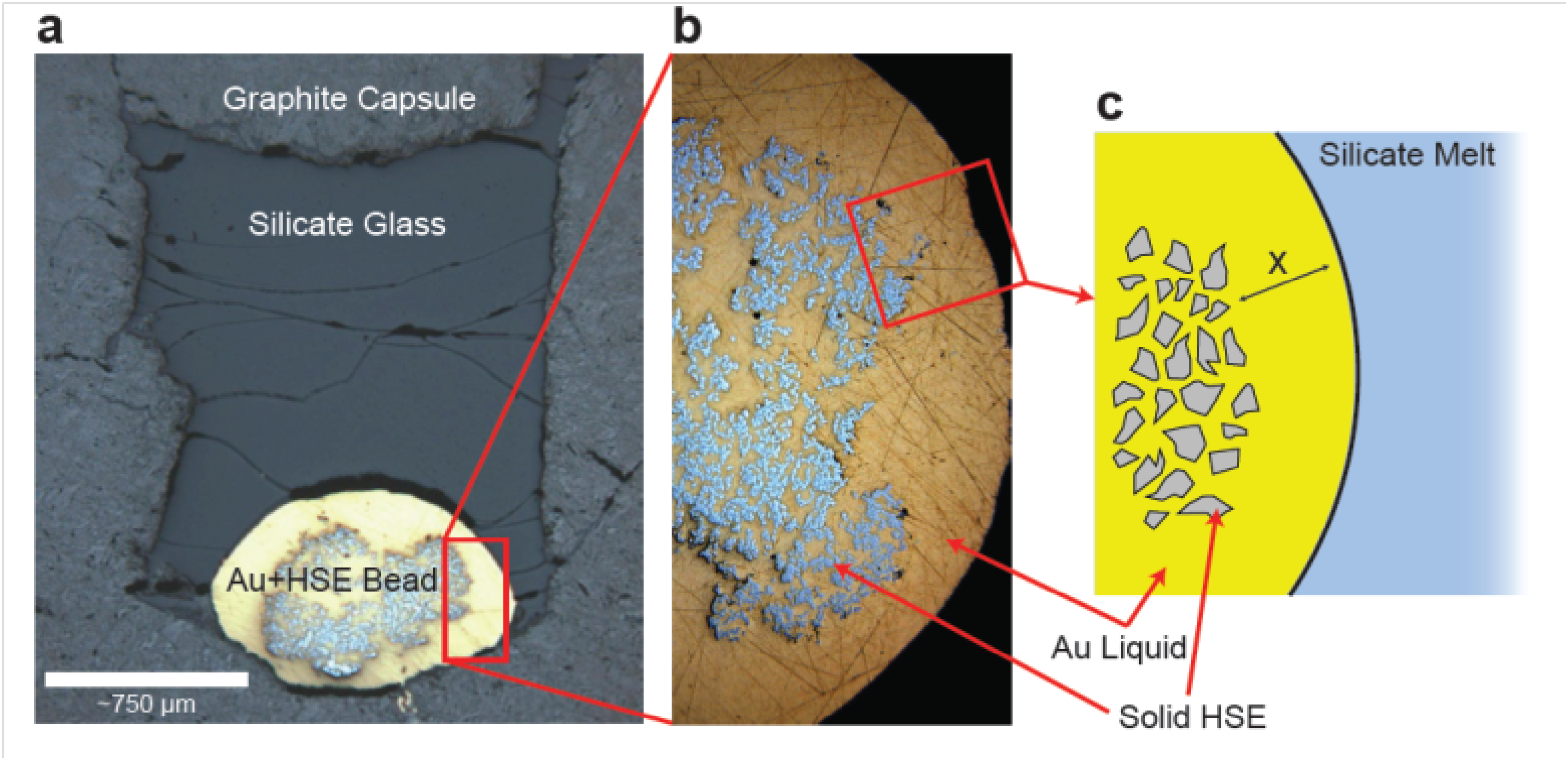
F. Origin of the large-scale distribution of the highly siderophile elements
Equilibrium during accretion of Earth’s core is predicted to variably deplete the silicate mantle in siderophile (iron-loving) elements, leaving behind large differences in their relative concentrations. Contrary to expectations, mantle abundances of the highly siderophile elements (HSE) are only 100x lower and similar in relative proportion to the terrestrial starting composition (chondrite). To produce the observed HSE composition of the mantle with an equilibrium model requires metal-silicate partitioning to be similar for all the HSE, and to have values much lower than previously measured. Recent work by others has shown that some HSE become less siderophile at extreme P and T, in support of the equilibrium hypothesis. In collaboration with Prof. Bill McDonough (U of Maryland), we did the initial measurements of the high temperature metal-silicate partitioning of Os, Ir and Au. This work was then followed up in more detail by Neil Bennett for his PhD dissertation, who also documented the behaviour of Re, Pt and Au. Neil was able to develop a method to suppress the formation of undissolved metal nuggets, which had been a problem in past experiments. This was a significant breakthough, allowing solubility to be measured at very reducing conditions. Results showed that Re, Os, Pt and Ir will be quantitatively removed from the mantle by core formation, whereas high levels of gold would remain. The resulting Re/Os would also produce a mantle Os isotopic composition which is too radiogenic compared to measured values. These new results make the equilibrium model untenable for the HSE, requiring a late veneer of meteoritic material following metal extraction. Owing to the strong temperature dependence of Au partitioning, the abundance of Au in the mantle may provide a geothermometer for core formation.
Relevant publications
1) Brenan, J.M. and McDonough, W.F., Core Formation and Metal-Silicate Fractionation of Osmium and Iridium from Gold. Nature Geoscience, 2, pp 798-801, 2009
2) Bennett, N. and Brenan, J.M. Controls on the solubility of rhenium in silicate melt: Implications for the osmium isotopic composition of Earth’s mantle. Earth and Planetary Science Letters, 361, pp 320-332, 2013.
3) Bennett, N., Brenan, J.M. and Koga, K.T. The solubility of platinum in silicate melt under reducing conditions: Results from experiments without metal inclusions. Geochimica et Cosmochimica Acta, 133, pp 422-442, 2014.
G. Origin of the association between chromite and the platinum group elements
Chromite is widely recognized to act as a collector for platinum group elements, but there is considerable uncertainty as to the nature of this process. Numerous observations have shown that the PGEs appear to be “mechanically” present as discrete grains of platinum group minerals (PGMs) included within magmatic chromite grains. That these grains could form at magmatic conditions had been confirmed by our previous work. However, the mechanism by which they form and the reason for their association with chromite was uncertain. In addition to inclusions, laboratory partitioning experiments had suggested some PGEs may dissolve to very high levels in spinel-structured minerals. However, all of the previous partitioning studies on iron-bearing systems had produced ferric-iron-rich spinels, whose cation site occupancy is very different from ferric-iron-poor chromite. The partitioning of PGEs into chromite had not been measured. Laboratory experiments were conducted to investigate both the “mechanical” and “dissolved” aspects of the chromite-PGE association. During these investigations, we made two significant discoveries. First, Craig Finnigan, as part of his PhD dissertation, was able to document a mechanism to produce PGMs at the magmatic stage as a result of micron-scale reduction in the silicate melt near growing chromite crystals (reduction-precipitation). This was an idea originally put forward by my colleague Jim Mungall. This effect can only occur for chromite, owing to its unique ability to incorporate Cr3+ and Fe3+ relative to Cr2+ and Fe2+. Entrapment of PGEs by this mechanism could significantly enhance the PGE content of growing chromite even if these elements are incompatible in the crystal structure. Results of the chromite-melt partitioning experiments done as part of Parisa Sattari’s M.Sc., then Finnigan’s PhD work, with follow-up experiments done by myself and then B.Sc. student Veronika Homolova, showed that Ir, Rh and Ru are much less compatible than previous measurements. From this data, we developed a new model for spinel-melt partitioning which incorporates the variation in site occupancies with composition, and provides a means to estimate the compatibility of PGEs into chromite-rich compositions. The combined results of the two studies suggest that reduction-precipitation may contribute most strongly to the chromite-PGE association at low fO2, but the dissolved PGE component would become more significant under more oxidising conditions.
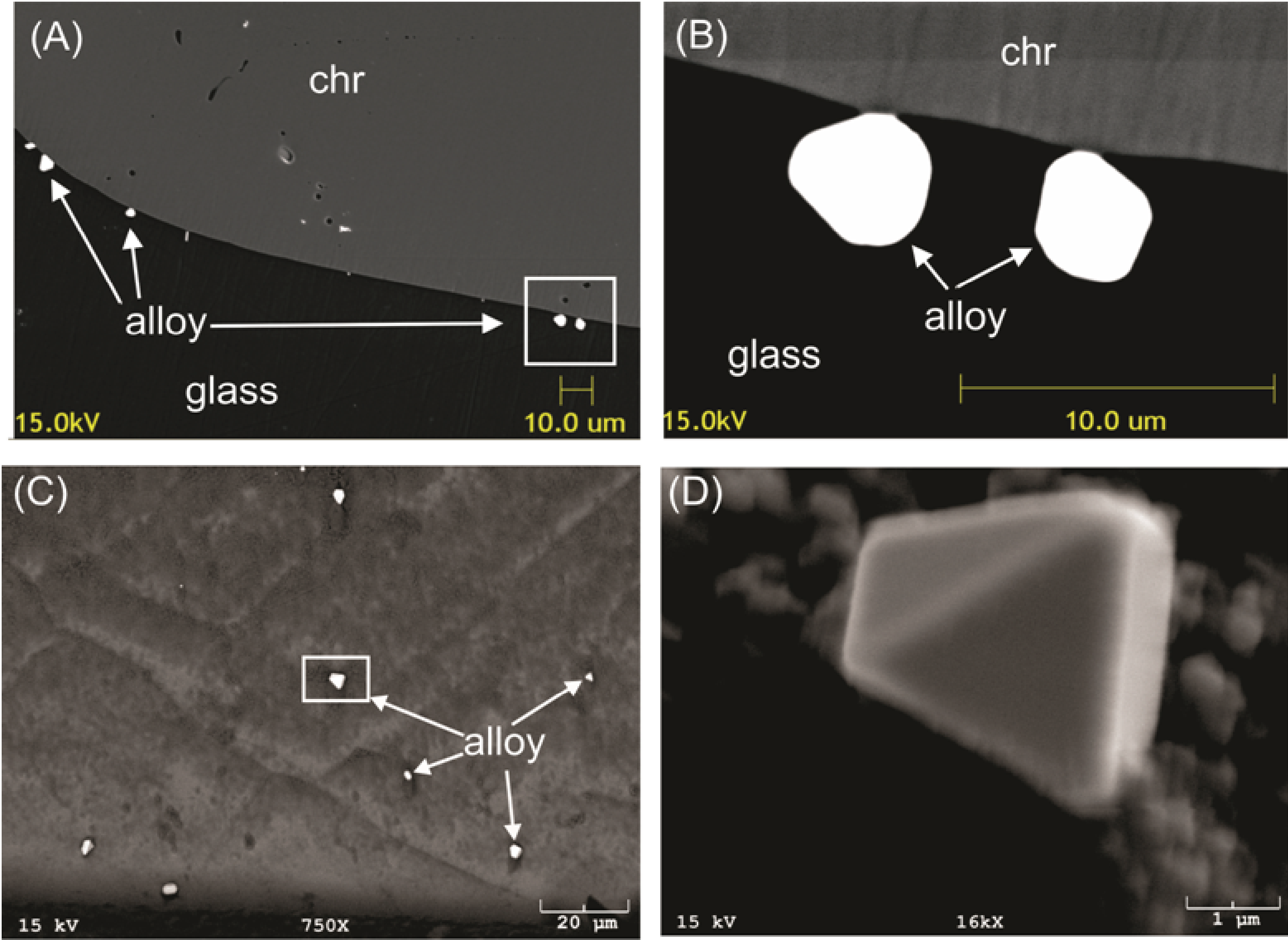
Relevant publications
1) Sattari, P., Brenan, J.M., Horn, I. and McDonough, W.F. Experimental constraints on the sulfide- and chromite-silicate melt partitioning behavior of rhenium and platinum-group elements, Economic Geology, 97: 385-398, 2002.
2) Finnigan, C.S., Brenan, J.M., Mungall, J.M. and McDonough, W.F., Experimental evidence for the co-precipitation of platinum group minerals and chromite by local reduction, Journal of Petrology, vol 49, pp 1647-1665, 2008.
3) Brenan, J.M., Finnigan, C.F., McDonough, W.F. and Homolova, V. Experimental constraints on the partitioning of the Ru, Rh, Ir, Pt and Pd between chromite and silicate melt. Chemical Geology, 302–303, pp 16–32, 2012.
H. Fractionation of highly siderophile elements and metal isotopes in planetary cores
To better understand the thermodynamic properties of metallic melts, former PhD. student Neil Bennett and I have explored the magnitude of element fractionation which may occur in molten Fe (+ S) subject to a high temperature thermal gradient. We discovered that the highly siderophile elements (HSE) are strongly separated into the hot and cold ends according to the sulfur content of the experiment. The magnitude of separation is different for each HSE, and in remarkable accord with experiments on solid metal-liquid metal partitioning, providing insight on the nature of element fractionation during planetary core formation from a single experiment. Follow-up Fe isotope analyses of run products done by Prof. Chip Lesher at U.C. Davis has revealed a significant fractionation for both Fe-S (~4 per mil/100 oC) and Fe (~1.5 per mil/100 oC) compositions, with the cooler end having higher values of δ56Fe. These are very large high temperature fractionations, allowing us to predict that outer core material within the thermal boundary layer at the lower mantle-outer core interface could be enriched in δ56Fe relative to chondritic abundances. Mixing of this material into the lower mantle could account for the enrichment in δ56Fe found in some mantle samples, providing new evidence for on-going core-mantle exchange.
Relevant publications
1) Brenan, J.M. and Bennett, N.R. Earth Planet. Sci. Lett., 298, pp 299-305, 2010
2) Lesher, C.E., Brenan, J.M., Barfod, G.H. and Glessner, J. Experimental constraints on Fe-isotope fractionation at the core-mantle boundary. V.M. Goldschmidt Conference, Montreal, June, 2012, with a paper currently in preparation.
I. The behaviour of chalcogens (Se, Te, As, Sb, Bi) during terrestrial accretion and magmatic differentiation
The elements selenium (Se) and tellurium (Te) are unique, geochemically, in terms of being volatile (50% condensation temperatures of 688 and 705 K, respectively), as well as chalcophile. These elements show anomalous (~30-fold) depletion in the silicate Earth, and relative abundances similar to CI chondritic meteorites. This behaviour is similar to the highly siderophile elements, which is interpreted to arise from strong partitioning into a core-forming metallic melt, followed by modest re-enrichment through late accretion (see section F). The question arises, therefore, do Se and Te also exhibit siderophile tendencies under appropriate conditions, or was the feedstock for Earth more depleted in these elements than previously supposed. For her PhD research, Lesley Rose-Weston measured the partitioning of S, Se and Te between metallic and silicate melt up to 2470 oC and 19 GPa over a range of oxygen fugacity. Resulting estimates for partitioning at core-forming conditions yields a silicate earth composition that is depleted by 100 (Se) to 100,000-fold (Te) lower than observed. Such results attest to the siderophile nature of these elements at the time of core-mantle differentiation, and supports a model for re-enrichment by ongoing accretion of meteorite material. Estimates of the composition of this late additive suggests it is more volatile-rich than previously supposed, possibly delivering nearly all of earth’s inventory for C and H. There is some uncertainty as to the veracity of this result, however, owing to the ambiguity in interpreting the mantle peridotite array in the context of a silicate earth composition. To establish a baseline for interpretation, PhD student Neva Fowler-Gerace and I have conducted experiments to measuring the partitioning of Se and Te amongst crystalline MSS, sulfide liquid, orthopyroxene, olivine and silicate melt. This data is used to develop models to simulate the compositional changes in residual peridotites, and sulfides, and to determine which, if any, characteristics of the mantle peridotite array are consistent with partial melting of a single, primordial source composition.
Experiments are also in progress to investigate the full suite of chalcogens, including Se, Te, Bi, Sb and As, in terms of their distribution amongst phases during the solidification of sulfide magmas. This is the thesis work of Ph.D. student Yanan Liu who has now measured partitioning between mono-sulfide- and intermediate- solid solution (ISS) and sulfide liquid at variable temperature and fixed oxygen and sulfur fugacity. These results can be used to construct realistic quantitative models of sulfide magma solidification, and predict the extent to which these elements will be concentrated in early-formed cumulates, or residual liquids. Results suggest that the chalcogens are incompatible in the initial MSS crystals, as well as later ISS, and will concentrate in the liquid phase. However, the strength of partitioning into the liquid is not too strong, such that enrichment of the chalcogens will not be significant until the last dregs of crystallization. Owing to the relatively high solubility of chalcogen-bearing PGMs in molten sulfide, the experimental results predict saturation of these phases only at the last stage of solidification, which is generally consistent with observation of natural samples.
Relevant publications
1) Rose-Weston, L.A., Brenan, J.M., Fei, Y., Secco, R.A. and Frost, D. Perspectives on Earth Differentitation from Metal-Silicate Partitioning of Te, Se, and S. Geochimica et Cosmochimica Acta., vol 73, 4598-4615, 2009.
2) Liu, Y and Brenan, J.M. Partitioning of platinum-group elements (PGE) and chalcogens (Se, Te, As, Sb, Bi) between monosulfide-solid solution (MSS), intermediate solid solution (ISS) and sulfide liquid at controlled fO2-fS2 conditions. Geochimica et Cosmochimica Acta, 159, pp 139-161, 2015.
3) Brenan, J.M. Se-Te fractionation by sulfide–silicate melt partitioning: Implications for the composition of mantle-derived magmas and their melting residues. Earth and Planetary Science Letters, 422, 45-57, 2015.
J. Elemental and isotopic partitioning of N and Li between minerals and aqueous fluid with implications for subduction-zone processes.
Subduction and progressive dehydration of oceanic crust in regions of tectonic convergence provides the necessary fluid flux to drive island arc volcanism. The behaviour of Li and N in these settings may provide some details of this process. Owing to the large relative mass difference amongst their isotopes, Li and N are subject to significant mass-dependent isotopic fractionation, due to both biogeochemical and inorganic processes. This fractionation serves to fingerprint subducted components, allowing their progress through the subduction cycle to be traced. Quantitative modeling of this behaviour is hindered by a lack of information on the elemental and isotopic partitioning of Li and N between fluid and any coexisting solid. Such data would provide information on the composition of fluids released by dehydration, and the capacity of the overlying mantle wedge to alter the fidelity of the slab signal. Natalie Caciagli-Warman’s PhD thesis focused on measurements of the mineral-fluid partitioning of Li, both elemental and isotopic, at high P-T conditions. Measurements of the Li isotopes were done in collaboration with Bill McDonough. The most significant result of that work is the demonstration that, because of moderate compatibility of Li for olivine, the mantle column above the slab strongly attenuates the slab Li signal if fluids traverse by porous flow. This provides an explanation for a paradoxical feature of arc magmas in which there is a lack of enrichment in Li, or shift from mantle Li isotopic composition, whilst other fluid-mobile elements are elevated. Only in cases where the slab-derived flux is large, or the slab-to-mantle pathways for Li are highly channelized, will the slab signal for Li be propagated to the zone of melting. Thus, Li provides information on the nature of the slab-to-mantle transfer process. More recent research, in collaboration with Prof. Gray Bebout (Lehigh University), has been to characterize the elemental and isotopic partitioning of N between muscovite and aqueous fluid. A major focus of this work is to develop quantitative models of fluid release during progressive metamorphism of the oceanic crust. These models can be tested against the observed variation in the elemental and isotopic composition of N in exhumed sections of oceanic crust, such as the Franciscan in coastal California. This work is a collaborative project funded by the U.S. National Science Foundation.
Relevant publications
1) Caciagli, N.C., Brenan, J.M., McDonough, W.F., and Phinney, D. Experimental constraints on Li partitioning and Li isotope fractionation during subduction zone dehydration. Chemical Geology, 280, pp 384–398, 2011.
K. Oxygen barometry from zircon-melt partitioning
Zircon is one of the most stable minerals in the surface environment, resisting chemical and physical abrasion, and often surviving multiple episodes of weathering, transport, burial, metamorphism and even partial melting. For these reasons, and due to relatively sluggish diffusion rates, zircon has been the mineral of choice for use in geochronology studies. Recent technical developments now allow for accurate determinations of zircon oxygen isotopic composition as well as trace element chemistry, providing a wealth of new information to assess zircon crystallization conditions. To fully interpret this data in terms of the characteristics of the zircon-forming melt requires appropriate experimental calibration. For example, recent experiments have provided a framework to interpret the temperature of zircon crystallization based on Ti content. We are pursuing a similar line of work to use the abundance of redox-sensitive elements in zircon to estimate oxygen fugacity. Amongst the rare-earth elements, both Ce and Eu are anomalous in that they may exist in other than the 3+ oxidation state at terrestrial magmatic conditions. Because the incorporation of elements into growing crystals is strongly coupled to ionic radius and charge, mineral-melt partitioning is likewise influenced by cation oxidation state. Consequently, the observation of pronounced “anomalies” in the abundances of cerium and europium in zircon can be ascribed to deviation from the “normal” partitioning of trivalent REEs, owing to the presence of Ce(IV) and Eu(II) in the zircon-forming magma. Since there may be some ambiguity to the interpretation of Eu anomalies as reflecting plagioclase fractionation, we are focusing our efforts on Ce. We have taken the approach of measuring melt speciation, then applying a well-established “elastic strain” model to develop an fO2 barometer based on zircon-melt partitioning. Duane Smythe’s PhD research was to conduct experiments to constrain the fO2-dependence of Ce(IV)/Ce(III) in various melt compositions using synchrotron-based measurements of the Ce M-edge XANES and wet chemical methods. He has used this data to develop a model of zircon-melt partitioning applicable to a variety of different crustal magma types, and tested it against natural samples whose fO2 has been independently constrained. These new data will vastly expand the amount of information one can obtain from a single zircon analysis, most notably the composition and redox state of zircon-forming magmas, including those that produced Earth’s earliest crust.
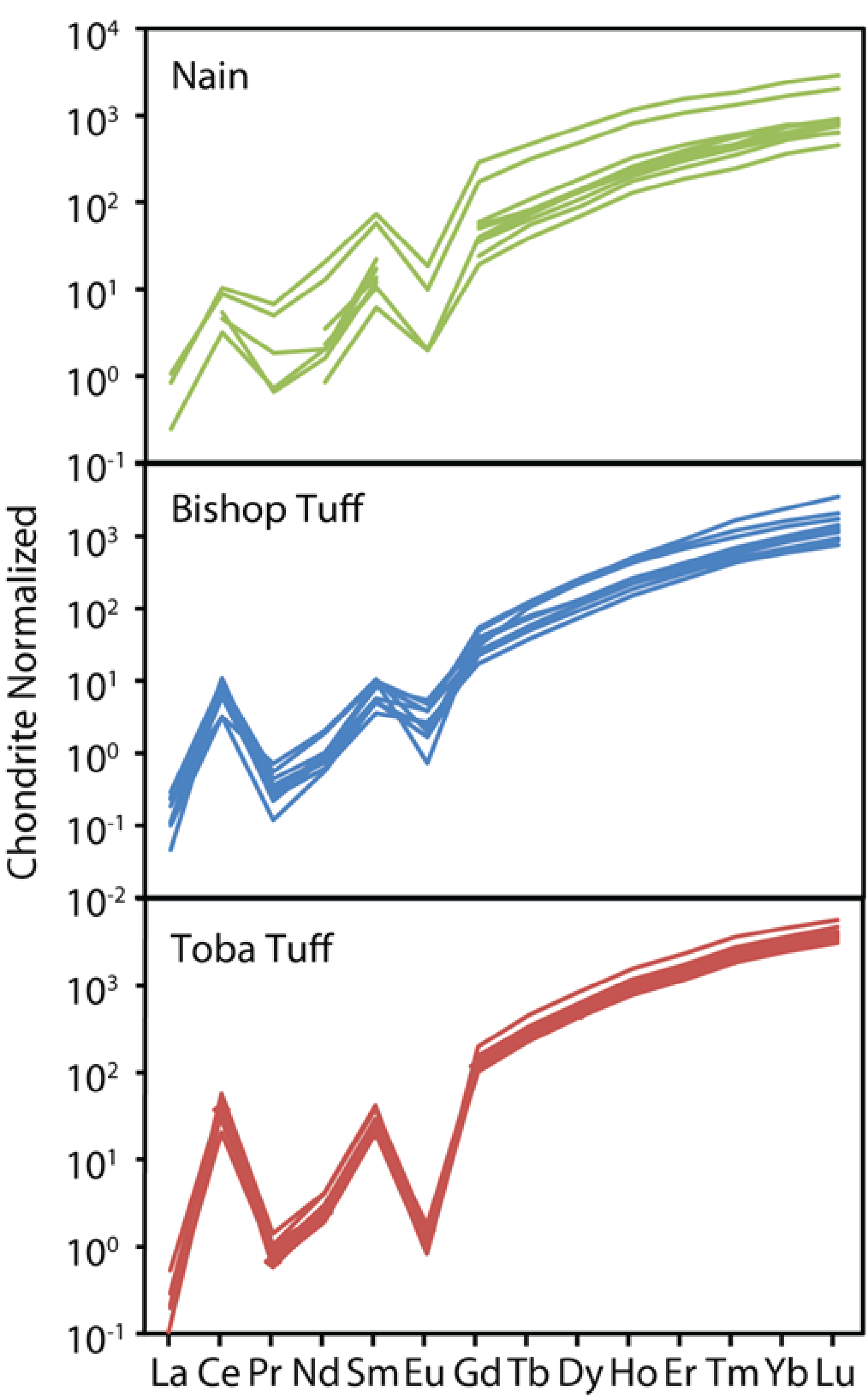
Relevant publications
1) Smythe, D. and Brenan, J.M, Henderson, G.S. Quantitative determination of cerium oxidation state in alkali-aluminosilicate glasses using Ce M4,5-edge XANES. Journal of Non-crystalline Solids, 378, pp 258-264, 2013.
2) Smythe, D. and Brenan, J.M. The effects of fO2 and melt composition on cerium speciation in silicate melts. In revision with Geochimica et Cosmochimica Acta.
An additional paper applying these results to oxygen barometry using zircon-melt partitioning is in preparation.



